Life Sciences faculty part of UCLA team studying how COVID-19 causes multiple organ failure
Researchers from the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA– including molecular, cell, and developmental biology professors Arjun Deb and Matteo Pellegrini, and microbiology immunology and molecular genetics professor, Jake Lusis– have received a $6.2 million grant from the National Institutes of Health to study how the SARS-CoV-2 virus causes damage throughout the body.
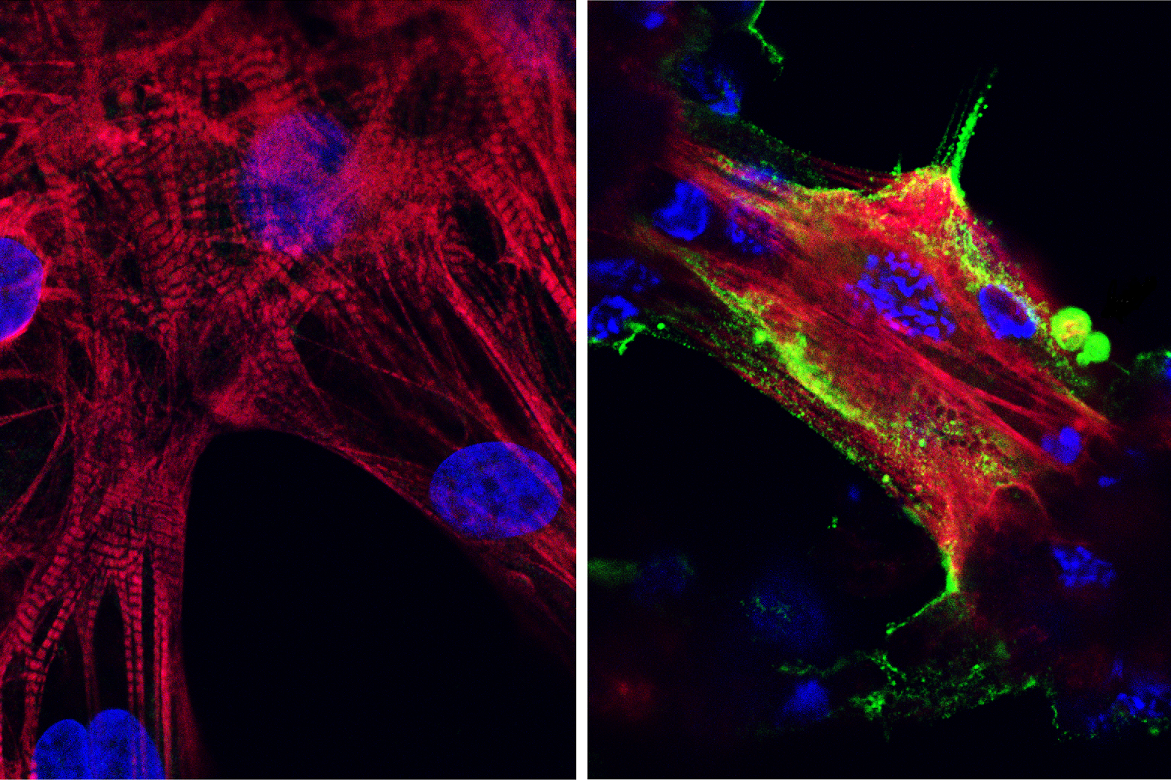
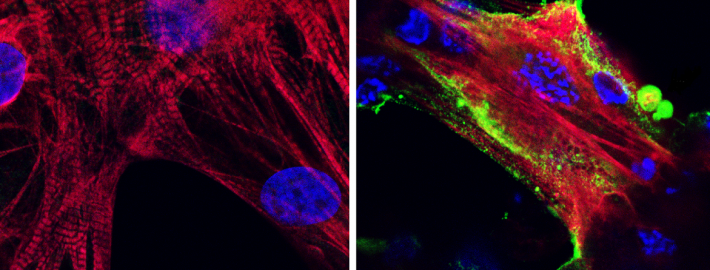
Microscope images showing (left) healthy heart muscle cells and (right) heart muscle cells that have been infected and damaged by the SARS-CoV-2 virus (in green). Photo Credit: UCLA Broad Stem Cell Research Center/JCI Insight
$6.2 million NIH grant to support UCLA study of how COVID-19 causes multiple organ failure
By Tiare Dunlap
Researchers from the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA have received a $6.2 million grant from the National Institutes of Health to study how the SARS-CoV-2 virus causes damage throughout the body.
The Director’s Transformative Research Award was presented through the NIH’s prestigious High Risk, High Reward program, which supports creative and unconventional approaches to major challenges in biomedical and behavioral research.
What makes SARS-CoV-2, the virus that causes COVID-19, so dangerous for some people is that although it is a respiratory virus, it can wreak havoc on almost any organ in the body. Studies have found that people who are hospitalized with COVID-19 and have damage to organs other than their lungs have a considerably higher risk for death. Even some people who recover from severe COVID-19 are left with long-term symptoms related to the damage to their other organs.
“It was a big puzzle at the start of the pandemic, and remains a big puzzle today, as to why a respiratory virus, which primarily affects the lungs, would cause such widespread damage throughout the body,” said Dr. Arjun Deb, director of the cardiovascular medicine research theme at the David Geffen School of Medicine at UCLA. “We need to figure out how this is happening in order to develop treatments to stop it.”
Deb is leading the UCLA research team, which also is made up of professors Vaithilingaraja Arumugaswami, Thomas Graeber, Jake Lusis and Matteo Pellegrini. The scientists will draw upon their combined expertise in cardiology, virology, metabolomics, genetics, genomics and computational biology to investigate how SARS-CoV-2 affects tissues throughout the body. To do so, they’ll conduct studies using a unique genetically engineered mouse model of COVID-19 that Deb and Arumugaswami developed in 2020.
Most mice used in COVID-19 research have to be modified to have the human ACE2 protein, which SARS-CoV-2 relies on to infect cells. But early studies of COVID-19 in mice relied on animals that only had the human ACE2 protein in their lungs. To better replicate cases in which COVID-19 affects other organs in humans, the researchers engineered mice to have human ACE2 in their hearts and other vital organs. They then infected these animals by injecting SARS-CoV-2 into their bloodstreams.
“The effects were startling,” said Arumugaswami, an associate professor of molecular and medical pharmacology at the medical school. “Within four days, the mice got very sick. Within seven days, they had lost 20% to 30% of their body weight and stopped moving.”
The mice had progressive organ failure that continued even after the virus had cleared from their bodies, mirroring the long-term damage in some people with COVID-19.
Thanks to Pellegrini, Graeber and Lusis’ expertise in single-cell genomics, computational modeling and metabolomics — the study of molecules called metabolites — the research in mice led the scientists to hypothesize that COVID-19 causes irreversible changes in an organism’s metabolism, the process by which cells create the energy they need to live.
Thanks to funding from the new grant, the group will seek to identify the genetic, metabolic and functional changes that occurred in each organ in both early and advanced stages of infection, and they’ll make their findings available to other researchers who are studying how the virus affects vital organs.
They also plan to compare the key changes they observe in mice with changes to blood and tissue samples from people with severe COVID-19. Their ultimate goal: to identify drugs that could prevent or disrupt the changes to cells’ metabolism, which could help reduce or eliminate the disease’s ability to damage vital organs.
“We suspect that the metabolic changes in the various organs will follow similar patterns,” said Graeber, a professor of molecular and medical pharmacology and director of the UCLA Metabolomics Center. “If that turns out to be the case, we will look for a single drug that could be widely applicable, regardless of which organs are affected.”
This article originally appeared in the UCLA Newsroom.


 Los Angeles Times Photographic Archive/ UCLA Library Special Collections
Los Angeles Times Photographic Archive/ UCLA Library Special Collections